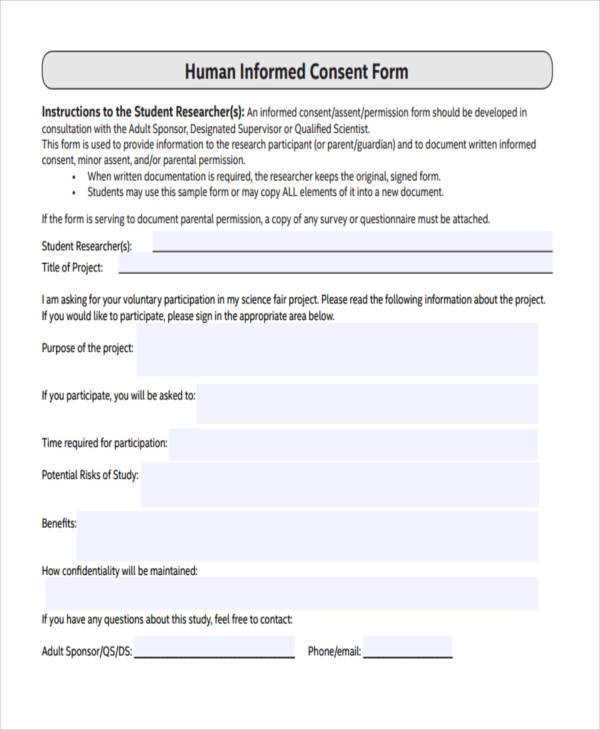
Human Informed Consent Form
Human Informed Consent Form - This effort can be lessened. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. When completing and irb submission in irbis, please fill in the. These consent form templates have been posted for your reference. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. Informed consent is the process of telling potential research participants about the key elements of a research study and what.
These consent form templates have been posted for your reference. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. This effort can be lessened. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Informed consent is the process of telling potential research participants about the key elements of a research study and what. When completing and irb submission in irbis, please fill in the. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed.
These consent form templates have been posted for your reference. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. This effort can be lessened. When completing and irb submission in irbis, please fill in the. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Informed consent is the process of telling potential research participants about the key elements of a research study and what. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort.
FREE 42+ Consent Form Samples in PDF MS Word Excel
When completing and irb submission in irbis, please fill in the. This effort can be lessened. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. Informed consent is the process of telling potential research participants about the key elements of a research study and what. This form is used to provide information.
FREE 15+ Informed Consent Form Samples, PDF, MS Word, Google Docs, Excel
This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. This effort can be lessened. Please note that these are templates developed by the who erc to assist the principal investigator.
SAMPLE INFORMED CONSENT FORM
Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. When completing and irb submission in irbis, please fill in the. Informed consent is the process of telling potential research participants about the key elements of a research study and what. A collection of informed consent, assent, and.
FREE 42+ Consent Forms in PDF MS Word Excel
When completing and irb submission in irbis, please fill in the. Informed consent is the process of telling potential research participants about the key elements of a research study and what. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. This effort can be lessened. Please note that these are.
Consent in Clinical Trials
These consent form templates have been posted for your reference. This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. When completing and irb submission in irbis, please fill in the. Informed consent is the process of telling potential research participants about the key elements of a research study and.
FREE 40+ Sample Consent Forms in PDF
This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. When completing and irb submission in irbis, please fill in the. Please note that these are templates developed by the who erc to.
FREE 33+ Consent Forms in MS Word
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort. Informed consent.
Form 4 human subjects informed consent
A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Informed consent is the process of telling potential research participants about the key elements of a research study and what. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their.
Human Informed Consent Form Fill Online, Printable, Fillable, Blank
When completing and irb submission in irbis, please fill in the. Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. Informed consent is the process of telling potential.
General Informed Consent Form Printable Consent Form
This form is used to provide information to the research participant (or parent/guardian) and to document written informed consent, minor. When completing and irb submission in irbis, please fill in the. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. These consent form templates have been posted for your reference..
These Consent Form Templates Have Been Posted For Your Reference.
Please note that these are templates developed by the who erc to assist the principal investigator in the design of their informed. A collection of informed consent, assent, and debriefing templates that can be used for your human participant research study. When completing and irb submission in irbis, please fill in the. Informed consent is the process of telling potential research participants about the key elements of a research study and what.
This Form Is Used To Provide Information To The Research Participant (Or Parent/Guardian) And To Document Written Informed Consent, Minor.
This effort can be lessened. Writing a consent form that uses plain language, and that is brief and clear, requires substantial effort.