Can Propane Form Isomers
Can Propane Form Isomers - Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Propane (c3h8) does not have any structural isomers. Find out why propane cannot have any isomers due to its. Structural isomers are compounds with the same molecular formula but different. Learn the definition and examples of structural isomerism in organic chemistry. As there is no double bond in. For isomers to exist, there should be a minimum of four carbons for branching to take place. In alkanes, isomerism arises when a particular compound. However iff there is hetero atom or any atom.
For isomers to exist, there should be a minimum of four carbons for branching to take place. Find out why propane cannot have any isomers due to its. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. However iff there is hetero atom or any atom. In alkanes, isomerism arises when a particular compound. Propane (c3h8) does not have any structural isomers. Structural isomers are compounds with the same molecular formula but different. Learn the definition and examples of structural isomerism in organic chemistry. As there is no double bond in.
Propane (c3h8) does not have any structural isomers. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Find out why propane cannot have any isomers due to its. As there is no double bond in. For isomers to exist, there should be a minimum of four carbons for branching to take place. However iff there is hetero atom or any atom. In alkanes, isomerism arises when a particular compound. Structural isomers are compounds with the same molecular formula but different. Learn the definition and examples of structural isomerism in organic chemistry.
SOLVED What are isomers? Write the isomers of butane and give their
However iff there is hetero atom or any atom. For isomers to exist, there should be a minimum of four carbons for branching to take place. Find out why propane cannot have any isomers due to its. Learn the definition and examples of structural isomerism in organic chemistry. Structural isomers are compounds with the same molecular formula but different.
What Are The Isomers Of Propanol? How Are They Determined?
In alkanes, isomerism arises when a particular compound. Structural isomers are compounds with the same molecular formula but different. As there is no double bond in. Learn the definition and examples of structural isomerism in organic chemistry. Propane (c3h8) does not have any structural isomers.
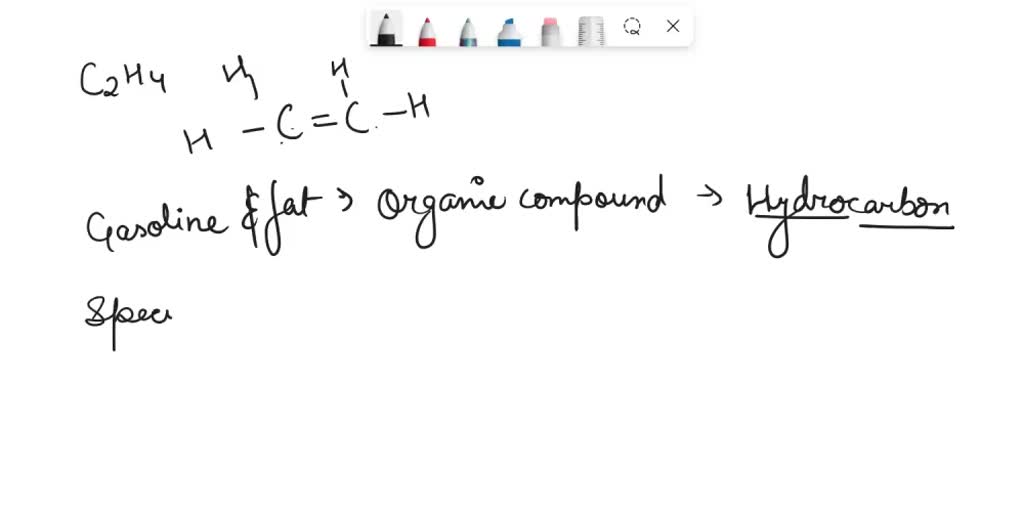
SOLVED 1) Draw structural formula for C2H4. 2) How are gasoline and
However iff there is hetero atom or any atom. Find out why propane cannot have any isomers due to its. In alkanes, isomerism arises when a particular compound. Learn the definition and examples of structural isomerism in organic chemistry. As there is no double bond in.
How many structural isomers can you draw pentane?
Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Learn the definition and examples of structural isomerism in organic chemistry. Propane (c3h8) does not have any structural isomers. For isomers to exist, there should be a minimum of four carbons for branching to take place. Find out why propane cannot have any.
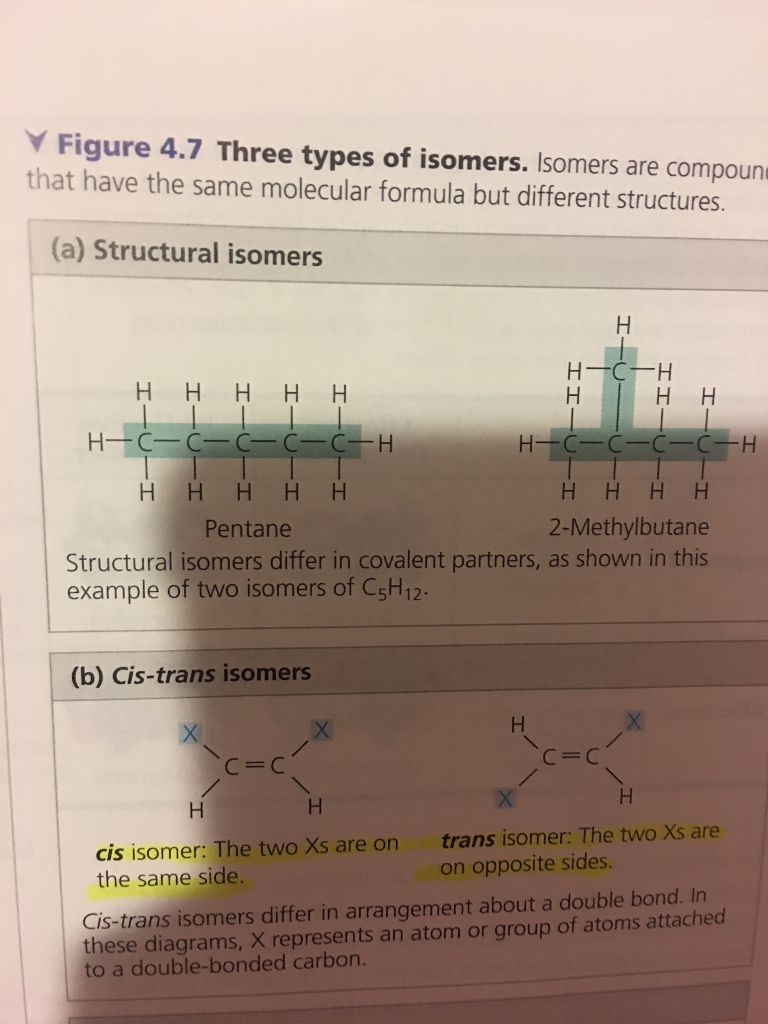
Chapter 4 Carbon and its Compounds NCERT Solutions for Class 10
Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. However iff there is hetero atom or any atom. In alkanes, isomerism arises when a particular compound. Propane (c3h8) does not have any structural isomers. Learn the definition and examples of structural isomerism in organic chemistry.
Una Breve Guida per Tipi di Isomeria in Chimica Organica Interesse
As there is no double bond in. For isomers to exist, there should be a minimum of four carbons for branching to take place. Propane (c3h8) does not have any structural isomers. However iff there is hetero atom or any atom. Find out why propane cannot have any isomers due to its.
Isomers vs Conformers CHEM123 chirp
Learn the definition and examples of structural isomerism in organic chemistry. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Structural isomers are compounds with the same molecular formula but different. For isomers to exist, there should be a minimum of four carbons for branching to take place. Propane (c3h8) does not.
CHEM Alkanes and Alkenes chemistry organic chemistr...
Structural isomers are compounds with the same molecular formula but different. For isomers to exist, there should be a minimum of four carbons for branching to take place. In alkanes, isomerism arises when a particular compound. Find out why propane cannot have any isomers due to its. However iff there is hetero atom or any atom.
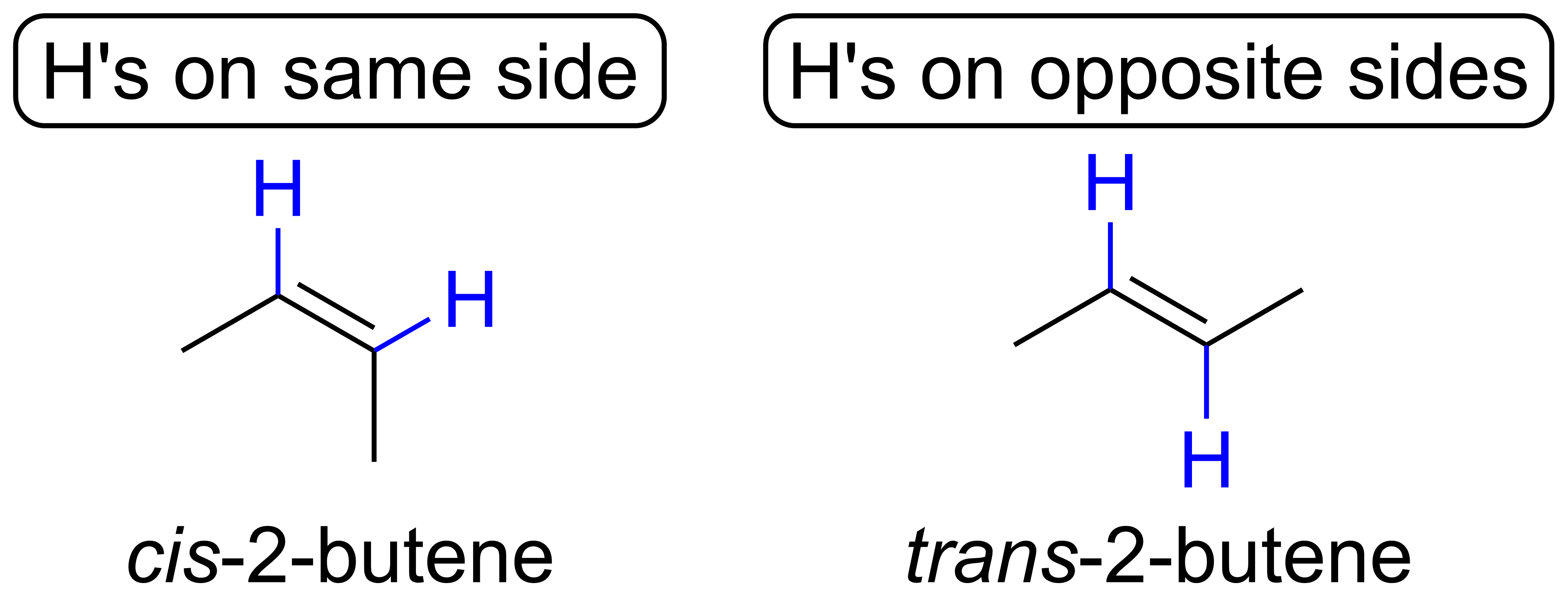
Solved See figures 4.5a and 4.7. Can propane (C3H8) form
Find out why propane cannot have any isomers due to its. In alkanes, isomerism arises when a particular compound. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. Structural isomers are compounds with the same molecular formula but different. As there is no double bond in.
Why are there no isomers of propane?
Propane (c3h8) does not have any structural isomers. However iff there is hetero atom or any atom. Find out why propane cannot have any isomers due to its. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. For isomers to exist, there should be a minimum of four carbons for branching to.
Find Out Why Propane Cannot Have Any Isomers Due To Its.
However iff there is hetero atom or any atom. Propane cannot form structural isomers because the three carbons atoms can attach only in a straight line. For isomers to exist, there should be a minimum of four carbons for branching to take place. Propane (c3h8) does not have any structural isomers.
Structural Isomers Are Compounds With The Same Molecular Formula But Different.
As there is no double bond in. Learn the definition and examples of structural isomerism in organic chemistry. In alkanes, isomerism arises when a particular compound.